Printable Multi Dose Vial 28 Day Expiration Calendar 2025 2026 1 What is a multi dose vial A multi dose vial is a vial of liquid medication intended for parenteral administration injection or infusion that contains more than one dose of medication Multi dose vials are labeled as such by the manufacturer and typically contain an antimicrobial preservative to help prevent the growth of bacteria
The checklist which is appropriate for both inpatient and outpatient settings should be used to systematically assess adherence of healthcare providers to safe injection practices Assessment of adherence should be conducted by direct observation of healthcare personnel during the performance of their duties Multi Dose Vial 28 Day Expiration Calculator ePAPER READ DOWNLOAD ePAPER TAGS december february january october april november august september expiration vial calculator gohcl gohcl Create successful ePaper yourself Turn your PDF publications into a flip book with our unique Google optimized e Paper software START NOW
Printable Multi Dose Vial 28 Day Expiration Calendar 2025 2026
Printable Multi Dose Vial 28 Day Expiration Calendar 2025 2026
https://menmd.com/wp-content/uploads/2019/09/SyringeandVile-01.jpg

Health Care Logistics Multi Dose Vial Labels W Expiration Date Right Way Medical
https://rightwaymed.com/wp-content/uploads/2021/01/HCL18369-min.png

How To Use Labels To Meet Vaccine Storage Guidelines United Ad Label
https://mailers.unitedadlabel.com/cdnr/92/acton/attachment/16611/f-e1b2df87-24dd-4cd7-9f46-02e5ef245c03/1/-/-/-/-/Multi Dose Label.jpg
About Injection safety is a basic expectation in patient care Injection safety includes safe handling of medications and using each needle and syringe for only one patient one time Unsafe injection practices have led to transmission of bloodborne pathogens such as hepatitis B and C HIV and bacteria ASHP Crosswalk of Guidances and Standards for Managing Single SDV and Multi Dose Vials MDV July 2013 This guide is an ASHP member resource prepared jointly by the Section of Pharmacy Practice Managers Advisory Group on Quality and Compliance and ASHP s Division on Medication Safety and Quality Contact
6 In the operative environment e g OR Holding PACU PCS and L D OR s multi dose vials may be used for a period of 24 hours 7 If the manufacturer s expiration date of recognized multi dose vials pens is prior to 28 days then the Management of multi dose vials of parenteral medications and vaccines USP and APIC now recommend that opened or punctured multi dose vials be used for no more than 28 days unless the manufacturer specifies otherwise Therefore The Joint Commission requires a 28 day expiration date for multi dose vials from the date of opening or puncture unless otherwise specified by the manufacturer
More picture related to Printable Multi Dose Vial 28 Day Expiration Calendar 2025 2026
Saxenda FDA Prescribing Information Side Effects And Uses
http://www.drugs.com/pro/images/3946d389-0926-4f77-a708-0acb8153b143/image-07.jpg
Pharmacists Deciding What To Do With Extra Vaccine Doses Left In Multi dose Vials Fox43
https://media.tegna-media.com/assets/WPMT/images/e10c1e33-4d73-4f36-bf2c-d087ad50df7e/e10c1e33-4d73-4f36-bf2c-d087ad50df7e_1920x1080.jpg

Thiamine Hydrochloride Injection USP
https://dailymed.nlm.nih.gov/dailymed/image.cfm?name=thi05-0001-04.jpg&id=478867
SDV A SINGLE DOSE VIAL SDV is approved for use on a SINGLE patient for a SINGLE procedure or injection SDVs typically lack an antimicrobial preservative Do not save leftover medication from these vials Harmful bacteria can grow and infect a patient DISCARD after every use SIZE DOES NOT MATTER MDV A MULTIPLE DOSE VIAL MDV All opened and or punctured multi dose vials should be discarded every 28 days unless otherwise specified by the manufacturer v Single dose vials must be discarded after each use and should never be used for multiple patients b Operative Environment OR Holding PACU PCS and L D OR s i Multi dose vials may be used for a period of
11 When multi dose vials MDV are used the label must be dated with a 28 day expiration date Opened MDVs must be stored according to manufacturer s recommendations Undated vials that are not properly dated will be discarded 12 When MDVs are used the following precautions to reduce potential for cross Overview This revision to the multi dose vial policy provides updated guidance on how to handle all opened multi dose vials of WHO pre qualified vaccines It outlines the conditions under which opened multi dose vials can be kept for 28 days and which must be discarded after 6 hours or at the end of the immunization session whichever comes

Federal Register Medicare And Medicaid Programs CY 2015 Home Health Prospective Payment
https://images.federalregister.gov/ER06NO14.009/original.png?1415261295
Infuvite Adult Multivitamin Injection 5 Dose Box 2 Vials Dose 10 Vials Box Refrigerated
https://www.mcguffmedical.com/content/images/thumbs/0015220_infuvite-adult-multivitamin-injection-5-dose-box-2-vialsdose-10-vialsbox-refrigerated.jpeg
https://www.cdc.gov/injectionsafety/providers/provider_faqs_multivials.html
1 What is a multi dose vial A multi dose vial is a vial of liquid medication intended for parenteral administration injection or infusion that contains more than one dose of medication Multi dose vials are labeled as such by the manufacturer and typically contain an antimicrobial preservative to help prevent the growth of bacteria

https://www.cdc.gov/injectionsafety/PDF/Safe-Injection-Checklist-P.pdf
The checklist which is appropriate for both inpatient and outpatient settings should be used to systematically assess adherence of healthcare providers to safe injection practices Assessment of adherence should be conducted by direct observation of healthcare personnel during the performance of their duties
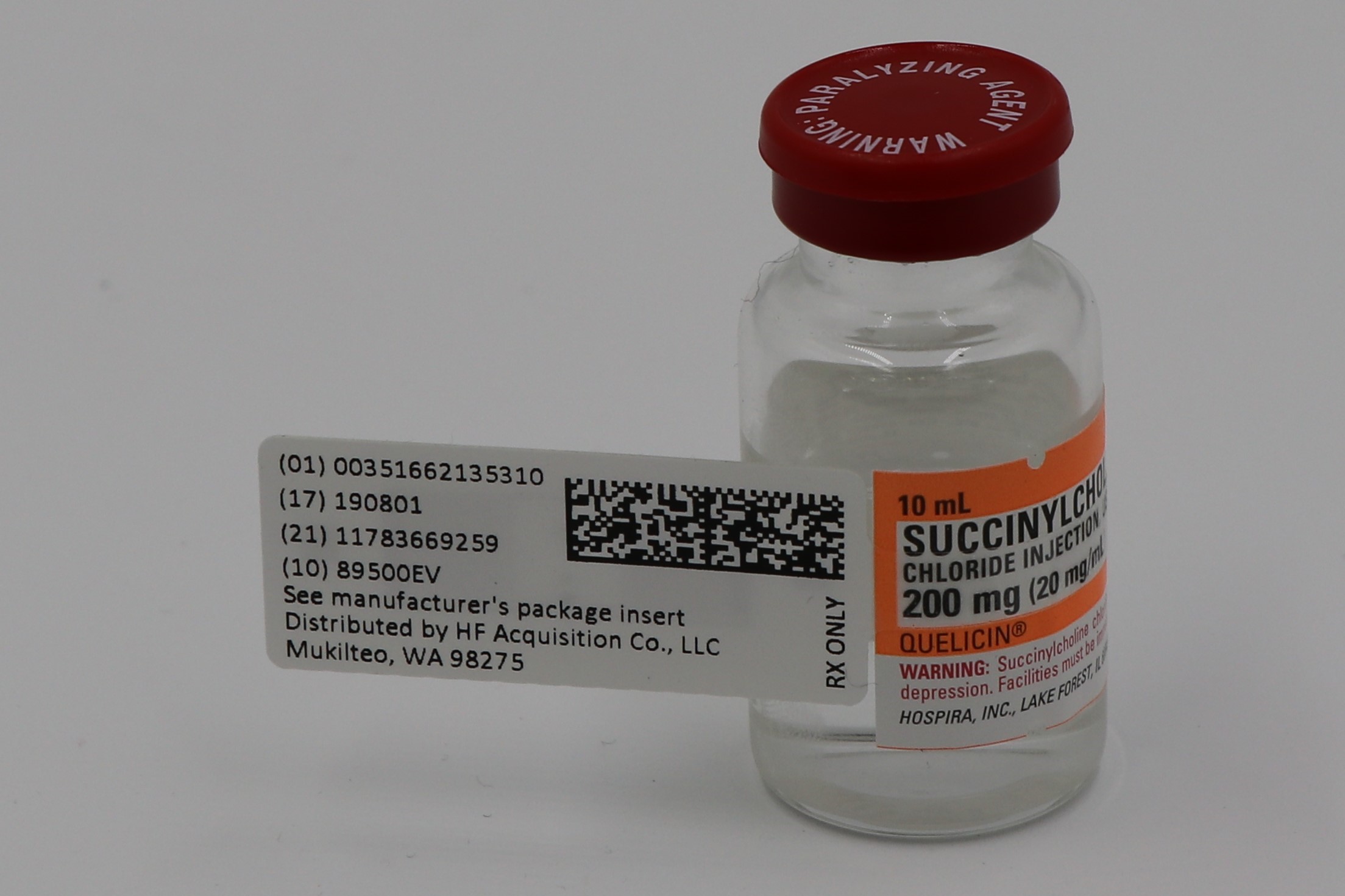
SUCCINYLCHOLINE CHLORIDE INJECTION USP 200mg 20mg mL 10mL VIAL

Federal Register Medicare And Medicaid Programs CY 2015 Home Health Prospective Payment

As Moderna Looks To Increase The Doses In Vaccine Vials The White House Announces An Expected
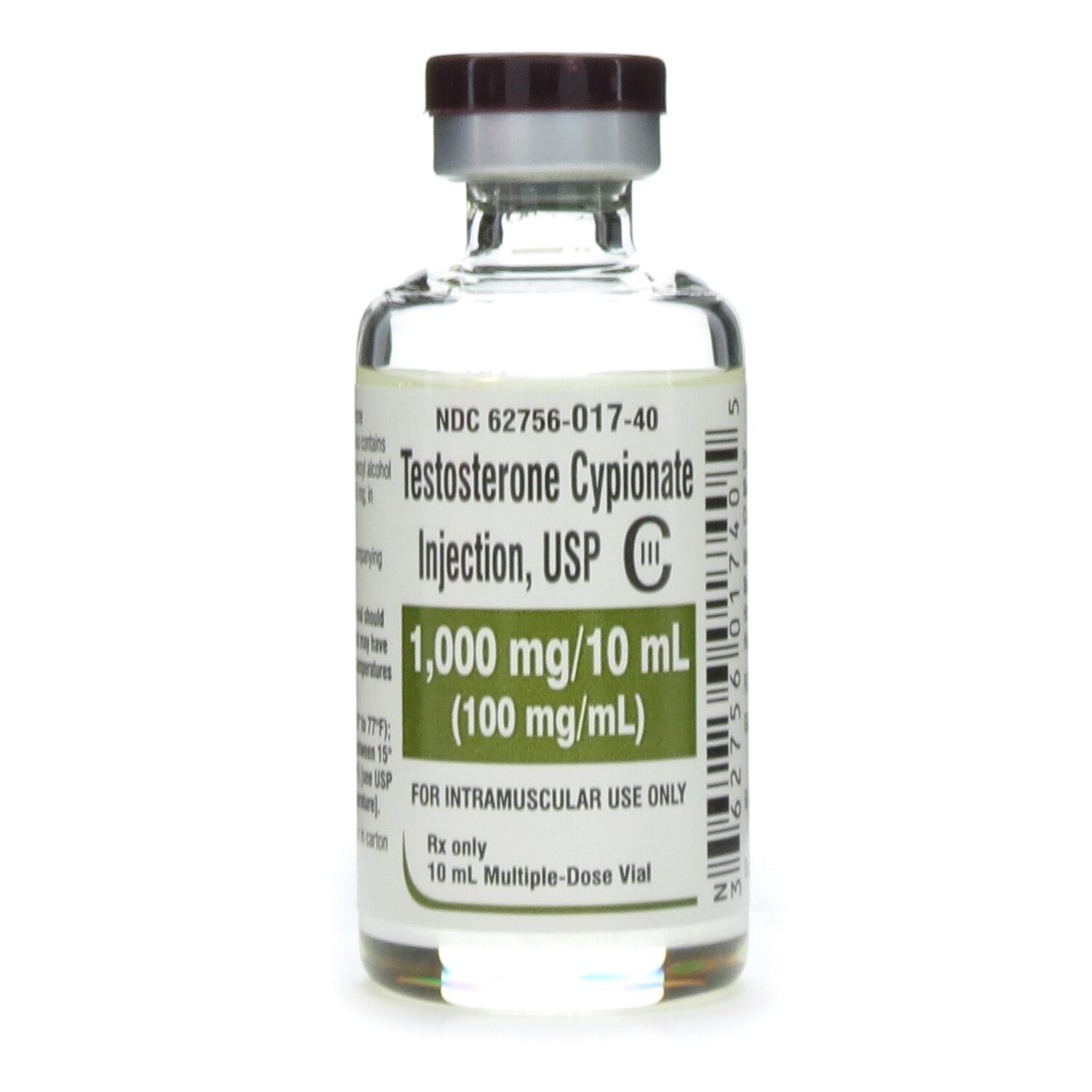
Testosterone Cypionate C III 100mg mL MDV 10mL Vial McGuff Medical Products

Multi Dose Vial 28 Day Expiration Calculator 2017 Printable Calendar Template 2020 In 2021

Multi Dose Vial 28 Day Expiration Calculator Image Https nomadedigital multi dose vial 28

Multi Dose Vial 28 Day Expiration Calculator Image Https nomadedigital multi dose vial 28

Multi Dose Vial 28 Day Expiration Calculator Image Calendar Printables Calendar Template

Multi Dose Vial 28 Day Calendar Image In 2021 Calendar Free Calendar Template Printable

Multi Dose 28 Day Calendar Template 2023
Printable Multi Dose Vial 28 Day Expiration Calendar 2025 2026 - USP and APIC now recommend that opened or punctured multi dose vials be used for no more than 28 days unless the manufacturer specifies otherwise Therefore The Joint Commission requires a 28 day expiration date for multi dose vials from the date of opening or puncture unless otherwise specified by the manufacturer