Multi Dose Vial 28 Day Expiration Calendar 2025 2026 If a multi dose has been opened or accessed e g needle punctured the vial should be dated with the last date that the product should be used expiration date and discarded within 28 days unless the manufacturer specifies a different shorter or longer date for that opened vial
A multi dose vial is a vial of liquid medication intended for parenteral administration injection or infusion that contains more than one dose of medication Multi dose vials are labeled as such by the manufacturer and typically contain an antimicrobial preservative to help prevent the growth of bacteria Therefore The Joint Commission requires a 28 day expiration date for multi dose vials from the date of opening or puncture unless otherwise specified by the manufacturer
Multi Dose Vial 28 Day Expiration Calendar 2025 2026

Multi Dose Vial 28 Day Expiration Calendar 2025 2026
https://www.pdffiller.com/preview/609/182/609182789/large.png

Health Care Logistics Multi Dose Vial Labels W Expiration Date Right Way Medical
https://rightwaymed.com/wp-content/uploads/2021/01/HCL18369-min.png

Project Firstline Resources For Multi Dose Vial Safety ANA
https://www.nursingworld.org/~49c632/globalassets/project-firstline/on-the-go-resources/youtube-screenshots/episode_8b.png
Yes Report breaches and lapses of injection safety and infections suspected of being associated with unsafe practices to your local health department If you are a licensed facility you must also report to a CDPH Licensing and Certification district office How Do I Follow Safe Injection Practices YF Vax Yellow Fever vaccine Sanofi 60 minutes after reconstitution Mar 2020 COVID 19 2023 2024 Formula for ages 6 months 4 years yellow cap vial Pfizer Unpunctured 10 weeks if stored in a refrigerator 2 8 C 36 46 F Punctured 12 hours after vial puncture Sep 2023
I Description Management of multi dose vials of parenteral medications and vaccines II Rationale This policy outlines the management of multi dose vials pens of injectable medications and vaccines in both institutional and clinic practices III Policy In general COPY the use of multi dose vials is discouraged SDV and Multi Dose Vials MDV July 2013 purposes It is not to be considered as medical legal or other professional contains general information only and may not reflect the most current the information contained herein and Contact David Chen Director Pharmacy Practice Sections and Section of Pharmacy Practice Managers DChen
More picture related to Multi Dose Vial 28 Day Expiration Calendar 2025 2026

How To Use Labels To Meet Vaccine Storage Guidelines United Ad Label
https://mailers.unitedadlabel.com/cdnr/92/acton/attachment/16611/f-e1b2df87-24dd-4cd7-9f46-02e5ef245c03/1/-/-/-/-/Multi Dose Label.jpg
Afluria Quadrivalent Flu Vaccine 2022 2023 Multi Dose Vial 1 X 5 ML Mountainside Medical
https://cdn.shopify.com/s/files/1/0996/0350/products/Afluria-Quadrivalent-Flu-Vaccine-2022-2023_-Multi-Dose-Vial-1-x-5-mL_1200x1200_crop_center.jpg?v=1669117880
Saxenda Dosage Guide Drugs
https://www.drugs.com/pro/images/3946d389-0926-4f77-a708-0acb8153b143/image-07.jpg
Overview This revision to the multi dose vial policy provides updated guidance on how to handle all opened multi dose vials of WHO pre qualified vaccines It outlines the conditions under which opened multi dose vials can be kept for 28 days and which must be discarded after 6 hours or at the end of the immunization session whichever comes Always prepare multi dose vial injections away from patient care spaces in a clean designated area Clean your hands before touching the vial Check the label to make sure it is a multi dose vaccine vial Check to make sure the vaccine is not expired or beyond use Look and see if the vaccine appears the way the vaccine maker tells you it should Use brand new sterile needles and syringes
Multi Dose Vial 28 Day Expiration Calculator ePAPER READ DOWNLOAD ePAPER TAGS december february january october april november august september expiration vial calculator gohcl gohcl Create successful ePaper yourself Turn your PDF publications into a flip book with our unique Google optimized e Paper software START NOW Overview This revision to the multi dose vial policy provides updated guidance on how to handle all opened multi dose vials of WHO pre qualified vaccines It outlines the conditions under which opened multi dose vials can be kept for 28 days and which must be discarded after 6 hours or at the end of the immunization session whichever comes
Pharmacists Deciding What To Do With Extra Vaccine Doses Left In Multi dose Vials Fox43
https://media.tegna-media.com/assets/WPMT/images/e10c1e33-4d73-4f36-bf2c-d087ad50df7e/e10c1e33-4d73-4f36-bf2c-d087ad50df7e_1920x1080.jpg
Nationwide Shortage Of Tuberculin Skin Test Antigens CDC Recommendations For Patient Care And
https://www.cdc.gov/mmwr/volumes/68/wr/social-media/mm6824a4_TBTestChange_21June19_Image_1200x675.jpg

https://www.jointcommission.org/standards/standard-faqs/home-care/medication-management-mm/000001529/
If a multi dose has been opened or accessed e g needle punctured the vial should be dated with the last date that the product should be used expiration date and discarded within 28 days unless the manufacturer specifies a different shorter or longer date for that opened vial

https://www.cdc.gov/injectionsafety/providers/provider_faqs_multivials.html
A multi dose vial is a vial of liquid medication intended for parenteral administration injection or infusion that contains more than one dose of medication Multi dose vials are labeled as such by the manufacturer and typically contain an antimicrobial preservative to help prevent the growth of bacteria

As Moderna Looks To Increase The Doses In Vaccine Vials The White House Announces An Expected

Pharmacists Deciding What To Do With Extra Vaccine Doses Left In Multi dose Vials Fox43

Thiamine Hydrochloride Injection USP

White DATE VIAL OPENED Expiration Labels United Ad Label
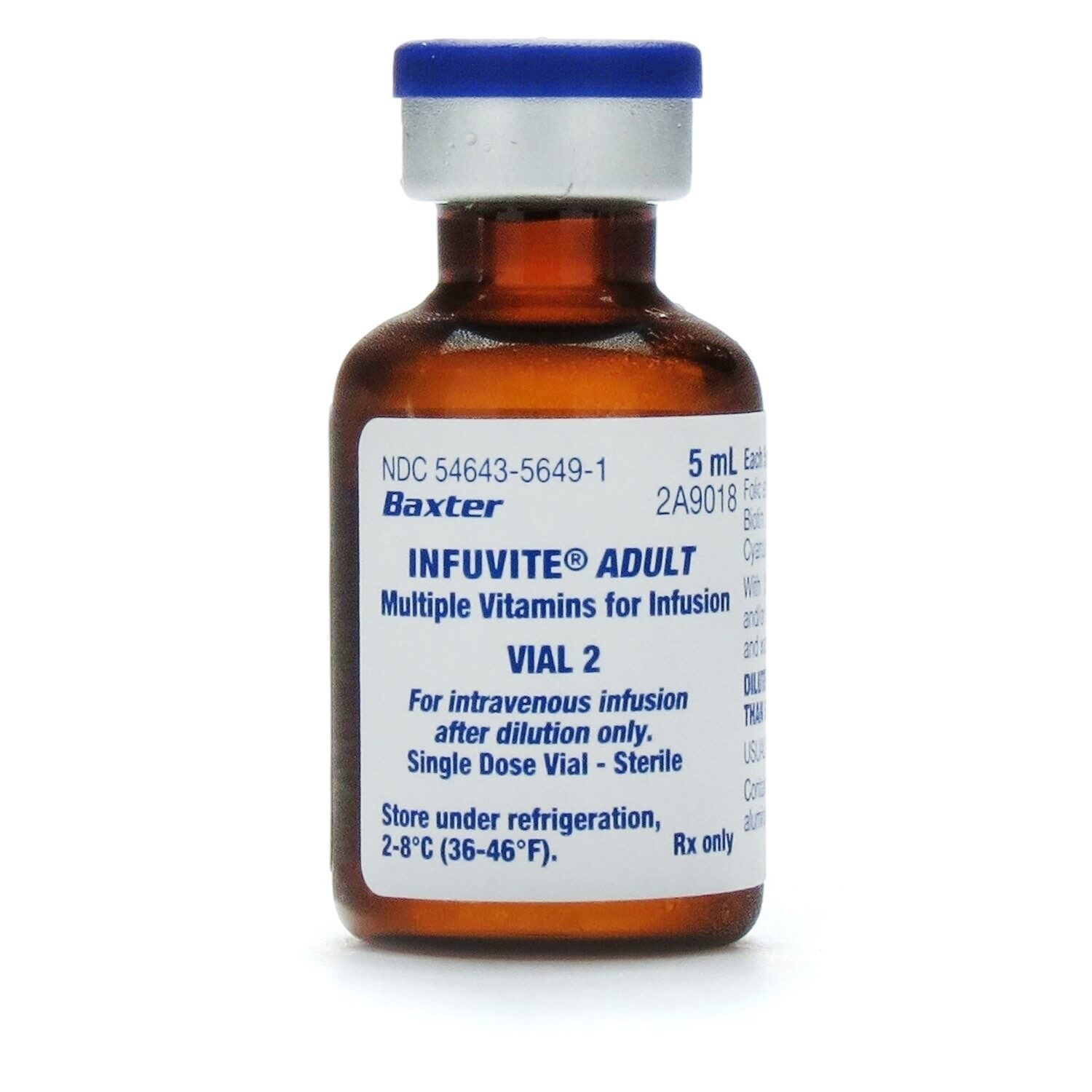
Infuvite Adult Multivitamin Injection 5 Dose Box 2 Vials Dose 10 Vials Box Refrigerated
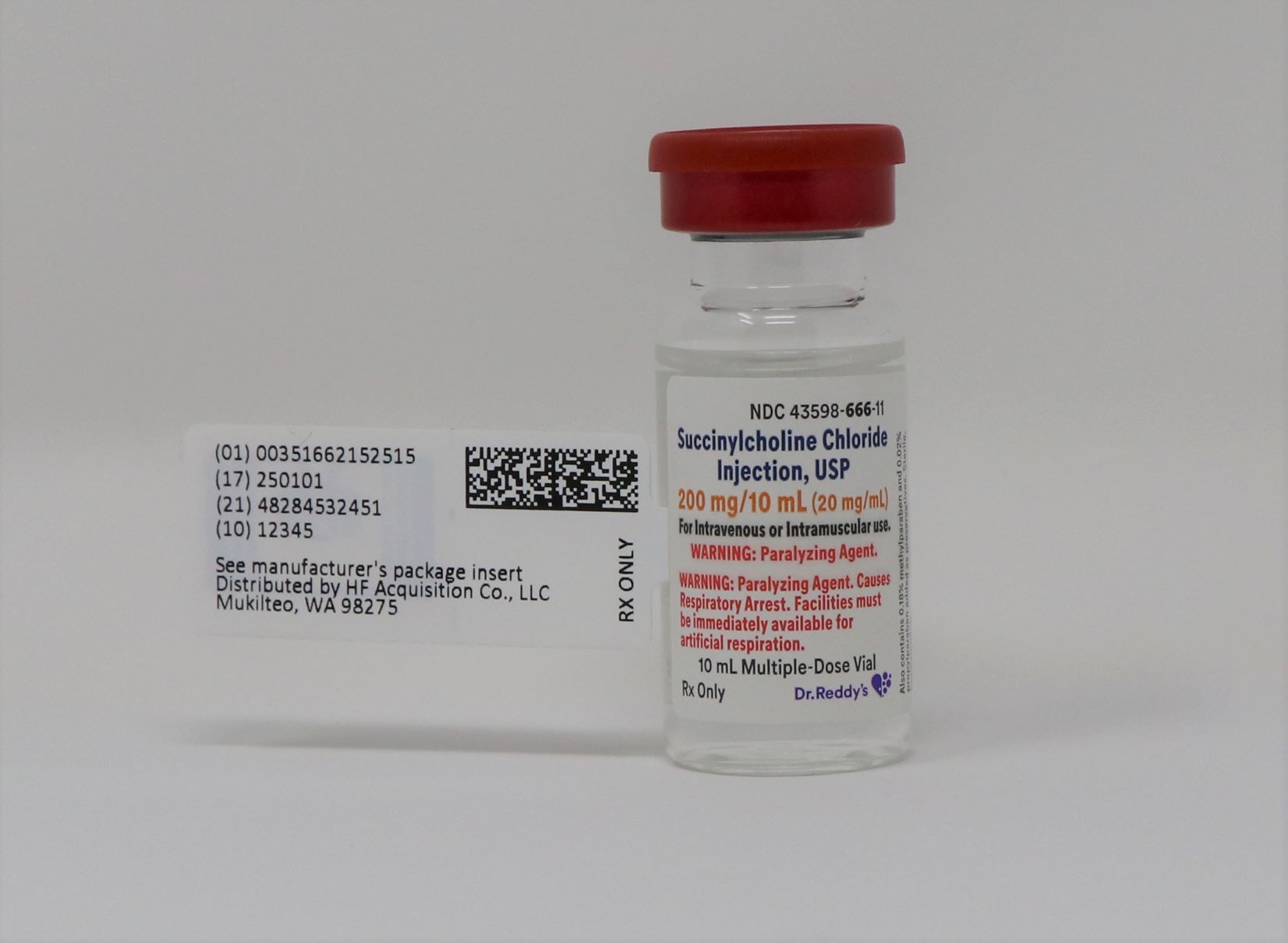
SUCCINYLCHOLINE CHLORIDE INJECTION USP 200mg 10mL 20mg mL 10mL VIAL

SUCCINYLCHOLINE CHLORIDE INJECTION USP 200mg 10mL 20mg mL 10mL VIAL

Federal Register Medicare And Medicaid Programs CY 2015 Home Health Prospective Payment
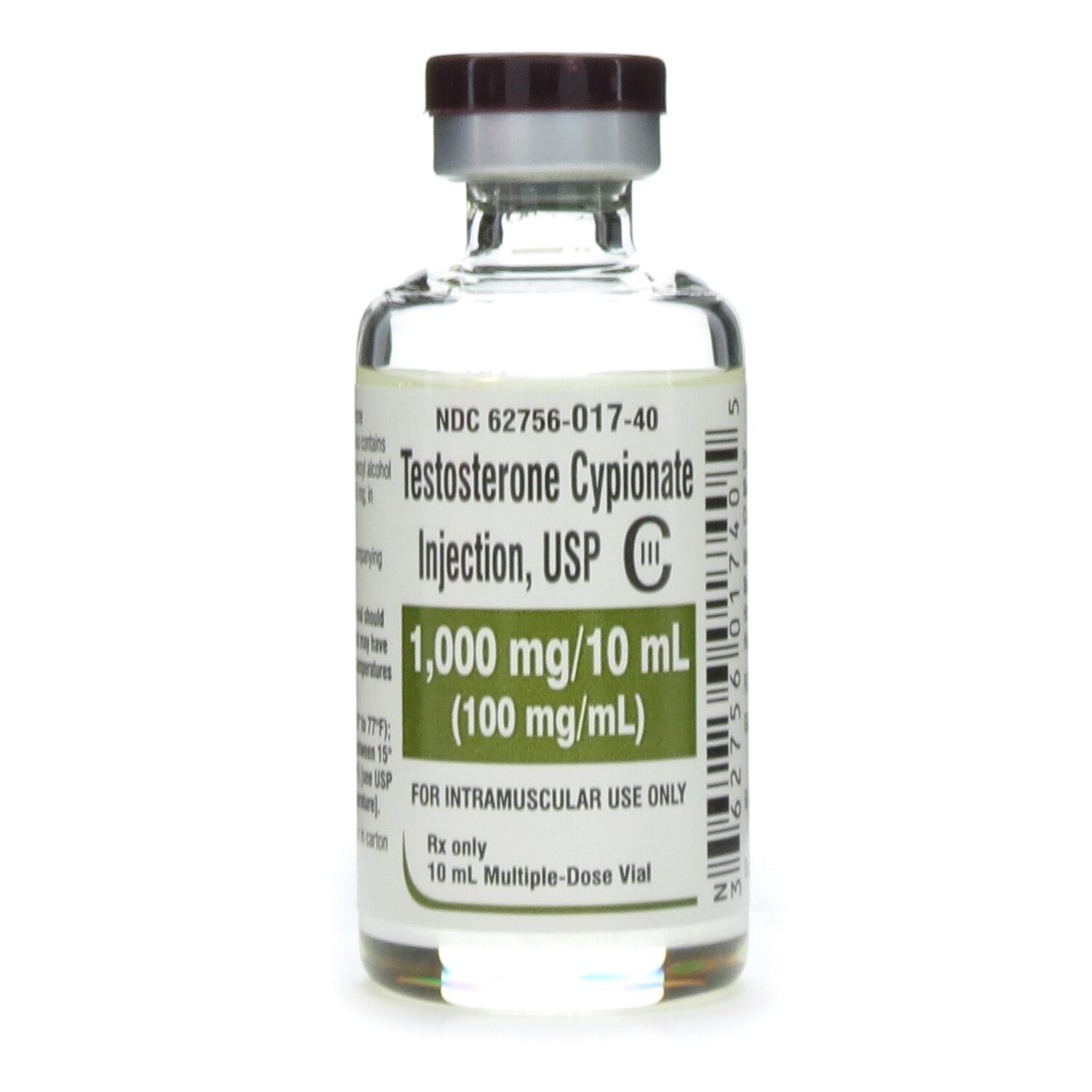
Testosterone Cypionate C III 100mg mL MDV 10mL Vial McGuff Medical Products

Multi Dose Vial 28 Day Expiration Calculator Image Https nomadedigital multi dose vial 28
Multi Dose Vial 28 Day Expiration Calendar 2025 2026 - Vial puncture Apr 2023 COVID 19 NVX CoV2373 Novavax 6 hours after vial puncture Mar 2023 CDC reference A multidose vial of vaccine that has been stored and handled properly and is normal in appearance can be used through the expiration date printed on the vial UNLESS otherwise stated in the manufacturer s product information